The first episode of the anime Dr.STONE has been released. It is a work that introduces various sciences, and uses science on a somewhat, or rather, rather, rather grand scale. The story itself is very interesting, and the characters are unique, but they are also very cool, cute, and strong, and I get the impression that they stand out. This time, I will focus on the science of the first episode and give a brief introduction.
Making gasoline from plastic bottle caps

A scene in the original where gasoline is made from plastic bottle caps. The hints are the molecular structure of polyethylene and the length of gasoline, cut off the hydrocarbon. That's what they said. I kind of understand what you're trying to say, so I looked it up.
Polyethylene: Making Gasoline from Plastic Bottle Caps

Molecular Formula
First, let's look at the molecular formula of polyethylene. It is written as (C2H4)n. The sticks coming out of the side of the C are, in very simple terms, a request for a handshake. There are two Cs, one of them holding each other's hand, and someone is holding the other hand. It's saying that n means there are a ton of C2H4s lined up on the side.
What is it used for?
Polyethylene is easy to process and inexpensive, so it is commonly used in mass-produced items. There seem to be many different types of polyethylene, and it is used in wraps, films, plastic buckets, and bathtubs. The plastic bags used at supermarkets are also polyethylene.
Making gasoline from plastic bottle caps What is gasoline?
Molecular formula
We say gasoline, gasoline, but in fact gasoline is a mixture. So we don't know the official composition formula of gasoline. Actually, it varies depending on the thing, but should we write it as a mixture of hydrocarbons with 4 to 10 carbon atoms? Actually, I read a lot about it, but it was difficult to understand. It seems that gasoline is a compound with n between 4 and 10 in CnH2n+2 (the 2 is not defined). There was a lot written, but it was difficult, so if you are interested in more, please ask someone who is good at science.
Gasoline is deep when you look into it
What color do you think gasoline is? In my mind, it was yellow, but it seems that it was intentionally colored to make it look like gasoline. Unemployed, transparent, what?
Making gasoline from plastic bottle caps Cutting up hydrocarbons
The idea
There are a lot of them lined up (C2H4). For example, let's say n is 100. Cut them up. So that it becomes 4-10. Then, lo and behold, gasoline is made! This is the theory. If you cut a plastic bottle cap, why does it become liquid? Does it become gasoline? Yes. It seems to. As long as you can cut it up.
How to cut it up
You don't physically cut it into pieces. You heat it. There seem to be various conditions, but it seems that it has to be heated to a fairly high temperature. I watched some experiment videos, etc., but if you're interested, please check it out on youtube.
Recycling in action
A while ago, there was an NPO that collected plastic bottle caps and donated them to people in poor countries. When they opened the caps, they were in the red and couldn't donate them. Plastic bottle caps are not actually suitable for chemical recycling. So they are promoting recycling by advocating various methods such as material recycling. It's a very good thing in terms of raising environmental awareness. But it's polyester. It doesn't seem rational.
What is nitric acid extracted from bat droppings?

Nitric acid is nitrogen dioxide dissolved in water, and its chemical formula is HNO3. Because it decomposes when exposed to light, it should generally be stored in the brown bottles you often see. It is liquid at room temperature because its melting point is -41.6 degrees and its boiling point is 82.6 degrees. It is very acidic, enough to dissolve metal. It is a dangerous substance, so generally, you should not touch it with your bare hands. If it gets on your hands, it will decompose when exposed to light, turn yellow, and it will not come off for a while. It is also not good for your body.
Nital is a real corrosive liquid
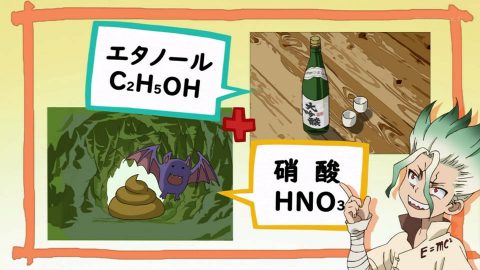
Nital, made from a mixture of nitric acid and ethanol, is a highly corrosive solution that is actually used. It is also used in metal corrosion experiments, and the fact that it contains nitric acid makes it seem dangerous. You should never touch it with your bare hands.
E=MC2

E=mc2 is drawn on Senku's clothes. This is the formula of energy E = mass m × speed of light c squared, which was concluded from Einstein's theory of relativity. This formula is very famous, but I'm sure there are many people who don't quite get it. Simply put, when mass decreases, energy decreases by the square of mc. (c is a constant.) In other words, the lost energy is converted into thermal energy, potential energy, and kinetic energy. It's a sad story, but the Hiroshima atomic bomb carried 64 kg of uranium, and it is said that the mass change (decrease in m) was 1 g. However, as this formula shows, it becomes 1 g x c2, and the energy that was lost from that is what caused that catastrophe.